Retail pharmacy certainly is not what it used to be. With the rise in large retail pharmacy purchases and partnerships with health insurers and providers, these organizations are contributing to more points of care than ever before. The Centers for Medicare & Medicaid Services (CMS) is the single largest payer for healthcare in the United States. Nearly 90 million Americans rely on healthcare benefits through Medicare, Medicaid, and the State Children’s Health Insurance Program (SCHIP).
The U.S. federal government is footing the bill for many prescription drugs and requires due diligence from healthcare organizations to ensure they are not hiring, contracting, or fulfilling prescriptions from excluded providers and entities. In this post, we will discuss some of the best practices for exclusion and preclusion monitoring for pharmacies and prescribers.
HHS-OIG Exclusion Authorities
According to the OIG Special Bulletin on the Effect of Exclusion and Participation in Federal Healthcare Programs, the law states that “The effect of an OIG exclusion is that no federal healthcare program payment may be made for any items or services furnished (1) by an excluded person or (2) at the medical direction or on the prescription of an excluded person.”
Section 1128A(a)(8) of the Social Security Act describes that “persons that order or prescribe items or services while excluded are subject to CMP liability when the excluded person knows or should know that a claim for the item or service may be made to a federal healthcare program.” To avoid liability, providers should ensure, at the point of service, that the ordering or prescribing physician is not excluded.
Excluded providers may refer a patient to a non-excluded provider if the excluded individual does not furnish, order, or prescribe any services for the referred patient, and the non-excluded provider treats the patient and independently bills federal healthcare programs for the items or services that he or she provides. Covered items or services furnished by a non-excluded individual to a federal healthcare program beneficiary are payable, even when an excluded provider referred the patient.
Prescriber Eligibility and Exclusion Monitoring
Pharmacies must take a proactive approach to ongoing exclusion list monitoring for prescribers they employ in addition to screening for each claim submitted to CMS for referring, ordering, and prescribing physicians. Excluded pharmacists and technicians cannot input prescription information for pharmacy billing, or fill prescriptions for drugs that are billed to a CMS federal healthcare program.
Many exclusions are the result of a license issue or revocation from a state medical board. For a comprehensive approach, primary source verification and license and credential monitoring are essential in addition to monitoring the OIG LEIE, SAM.gov, and all available state Medicaid lists.
Those looking to be reimbursed by CMS must have a valid National Provider Identifier (NPI) and be enrolled in Medicare, or provide an Opt Out Affidavit if they do not wish to participate.
DEA Registration for Prescribers
The HHS OIG Special Advisory Bulletin also addresses DEA registration and emphasizes the explicit need for exclusion screening by stating, “Some excluded practitioners will have valid licenses or Drug Enforcement Agency (DEA) numbers. Therefore, it is important not to assume that because a prescription contains a valid license number or DEA number, the practitioner is not excluded.”
The OIG goes on to say, “In some cases, pharmacies and laboratories rely on Medicare Part D plans and/or state agencies to ensure that prescribers are not excluded through, for example, computer system edits. These alternative screening mechanisms may effectively identify excluded individuals and prevent the pharmacies or laboratories from submitting claims for services ordered or prescribed by excluded individuals.
However, pharmacies and laboratories that rely on a third party to determine whether prescribers are excluded should be aware that they may be responsible for overpayments and CMPs relating to items or services that have been ordered or prescribed by excluded individuals.”
CMS Preclusion List and OIG LEIE
The Preclusion List from the Centers for Medicare and Medicaid Services (CMS) is a list of providers and prescribers who are precluded from receiving payment for Medicare Advantage (MA) items and services or Part D drugs furnished or prescribed to Medicare beneficiaries. The list was created to replace the Medicare Advantage (MA) and prescriber enrollment requirements, and ensure patient protections and safety from prescribers and providers identified as bad actors.
Who is on the CMS Preclusion List?
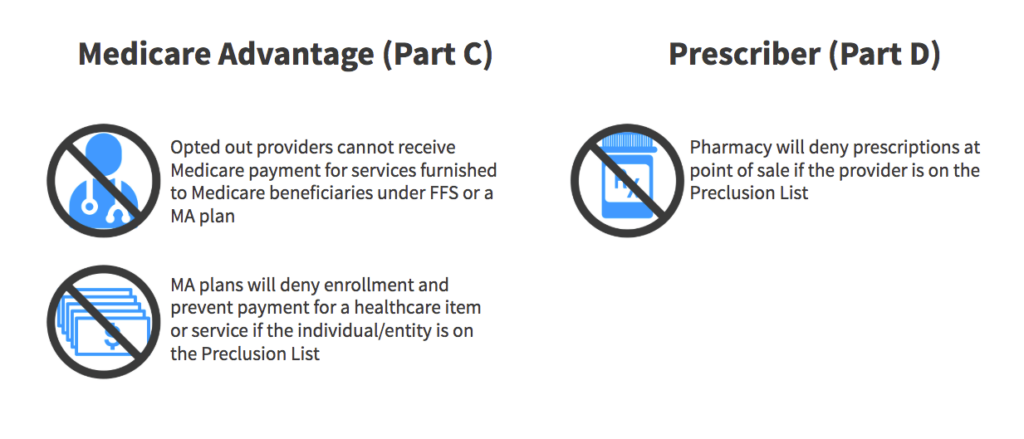
Pharmacy Benefit Managers are required to reject a pharmacy claim for a Part D drug if the individual who prescribed the drug is included on the list as determined by the Centers for Medicare and Medicaid Services (CMS).
Use of the Preclusion List does not eliminate or address the responsibility for Medicare plans MA and Part D plans to validate that providers are not included in the Office of Inspector General (OIG) exclusion file.
The Preclusion List and OIG LEIE overlap in the sense that excluded providers will be on the Preclusion List but precluded providers who are not excluded will not be on the OIG LEIE. It is easy for some confusion to set in because of the nature of common healthcare phrases or terms being used in multiple settings, but meaning completely different things. For example, the state exclusion list for Pennsylvania is called, “Pennsylvania Medicheck Precluded Providers List” as well as other instances providers being considered, “precluded” on certain state exclusion/sanction lists.
Initial CMS Preclusion List Insights
Since the CMS Preclusion list was implemented in January 2019, when comparing our data between exclusion and preclusion lists, 48% of precluded providers were also found to be excluded on any state or federal source (OIG LEIE, SAM.gov, All Medicaid State Lists). Our report also showed that 27 different sources (federal and state) were represented with the overlap between each list.
On average, we’ve seen that it takes 173 days for a state exclusion to make it to the OIG LEIE, even though states are required to report within 30 days from HHS OIG. In addition to exclusions, there were a few other stats that stood out to us – 7 NPIs on the list were found Inactive on January’s Preclusion List and 1 entity was found on the Medicare Opt-Out List as well.
HHS-OIG Enforcement
There are many examples of prescriber and pharmacy owner fraud. It’s important to recognize the most vulnerable situations and opportunities for fraud, waste, and abuse to occur and where healthcare organizations are responsible to mitigate risk and protect patients and beneficiaries.
HHS OIG identified that Medicare Part D does not have three key tools to protect against pharmacy fraud that are available in other parts of Medicare: pharmacy enrollment, revocation, and preclusion.
These three tools apply to pharmacies only when they bill Parts B or C, not when they bill Part D. OIG continues to recommend that CMS require pharmacies that bill Part D to enroll in the Medicare program. This was an integral part of a previous recommendation that CMS has yet to implement. CMS concurred with all three of the new recommendations from OIG, which were:
- Allow revocation of Medicare enrollment for inappropriate billing of Part D
- Include on the Preclusion List pharmacies that inappropriately bill Part D
- Apply the Preclusion List payment prohibitions to pharmacies and other providers that dispense Part D drugs
Government Healthcare Data Inefficiencies
With so many connection points that need to be made when it comes to federal healthcare programs, keeping up with many sources of information and organizational compliance tasks can be overwhelming. Further, most of these databases do not contain the same information or report at different times. We’ve made it our mission to step into the data gaps and build some common ground.
Our team has built a collection of automated solutions to help address these data concerns as they pertain to monitoring provider credentials, sanctions screening, provider eligibility, vendor engagement, MA ownership, management attestation, and more. Our goal is to help partner with healthcare organizations to include and support the best providers to deliver excellent patient care and pharmaceutical services.
Because pharmacy networks and retail locations are engaging in more points of care, we’ve recognized new opportunities and urgency in mitigating risk for hiring and managing clinical staff, and ensuring no false claims are submitted from these organizations.
We guarantee precision and accuracy with enriched data to fill in the gaps of prescriber eligibility concerns, in real-time. This helps give your organization peace of mind, knowing that each line of business is covered when it comes to monitoring federal healthcare programs.